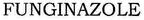Company Information
CIN
Status
Date of Incorporation
13 November 2000
State / ROC
Delhi / ROC Delhi
Industry
pharmaceuticals: antibiotics, endocrine products
Sub Category
Non-govt company
Last Balance Sheet
Last Annual Meeting
Paid Up Capital
2,500,000
Authorised Capital
10,000,000
Directors
Past Directors
Patents
"A solid iipid nanoparticles having a drug or plurality of drugs encapsulated therein".
This invention relates to a solid Iipid nanoparticles having a drug or plurality of drugs encapsulated therein and having sustained controlled release of the drug and there is provided a process for the preparation of solid Iipi...
The present invention is related to the development of a novel inter and intra multilamellar vesicular composition containing and antipsoriatic drug dithranol.
demands so. Thus, the product has improved the dithranol therapy along with some additive advantageous features.
A microemulsion organogel system for site-specific delivery of cyclosporin composed of gelator molecules, a polar/aqueous phase, and appropriate organic solvents wherein cyclosporin is in liposolubilzed state in the organogels.
A novel formulation for treating fungal infections comprising Cholesterol
containing Nanosomal Amphotericin B in a Saline Suspension
This invention relates to a process for the preparation of alginate nanoparticles having one or more drugs comprising singularly or a combination of rifampicin (RIF), isoniazid (INH), pyrazinamide (PZA) and ethambutol (EMB) encapsulated therein comprising preparing an aqueous solution of said drugs or drugs along wi...
This invention relates to a process for the preparation of poly-DL-lactide-co-glycolide nanoparticles (PLG-NP) having atleast two different active substances with different or same solubility properties encapsulated therein comprising steps of: preparing an aqueous solution of hydrophilic drug in distilled water (DW...
Trademarks
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Pharmaceutical, Veterinary And Sanitary
Preparations; Dietetic Substances Adapted For
Medical Use, Food For Babies; Plasters, Materials
For Dressings; Materials For Stopping Teeth,
Dental Wax; Disinfectants; Preparation For
Destroying Vermin; Fungicides, Herbicides."
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Prearations
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Anticancer Drug.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Anti Tuberculosis Drugs.
[Class : 5] Medicinal And Pharmaceutical Praparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And
Preparations.
Pharmaceutical
[Class : 5] Pharmaceuticals Preparations, Antibiotic Drug And Teicoplanin.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Application form not digitised
[Class : 5] Medicinal & Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Liposomal Amphotericin B.
[Class : 5] Pharmaceutical Preparation And Healthcare Products.
[Class : 5] Pharmaceutical, Veterinary And Sanitary Preparations; Dietetic Substances Adapted For
Medical Use, Food For Babies; Plasters, Materials For Dressings; Materials For Stopping Teeth, Dental Wax; Disinfectants; Preparation For Destroying Vermin; Fungicides, Herbicides.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticals Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticals Preparations
[Class : 5] Itraconazole.
[Class : 5] Pharmaceutical, Veterinary And Sanitary Preparations; Dietetic Substances Adapted For Medical Use, Food For Babies; Plasters, Materials For Dressings; Materials For Stopping Teeth, Dental Wax; Disinfectants; Preparation For Destroying Vermin; Fungicides, Herbicides.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations; Sanitary Preparations For Medical Purposes; Dietetic Food And Substances Adapted For Medical Or Veterinary Use, Food For Babies; Dietary Supplements For Human Beings And Animals; Plasters, Materials For Dressings; Material For Stopping Teeth, Dental Wax; Disinfectants; Preparations For Destroying Vermin; Fungi...
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations; Sanitary Preparations For Medical Purposes; Dietetic Food And Substances Adapted For Medical Or Veterinary Use, Food For Babies; Dietary Supplements For Human Beings And Animals; Plasters, Materials For Dressings; Material For Stopping Teeth, Dental Wax; Disinfectants; Preparations For Destroying Vermin; Fungi...
[Class : 5] Disinfectants And Antiseptics, Sanitizers, Hand Sanitizing Preparations, Alcohol Based Antibacterial Skin Sanitizer Gels.
[Class : 5] Pharmaceuticals, Medical Preparations And Fungicidal Preparations.
Awaiting Reply to Examination Report
[Class : 5] Pharmaceutical, Veterinary And Sanitary Preparations, Dietetic Substances Adapted For Medical Use, Food For Babies, Plasters, Materials For Dressings, Material For Stopping Teeth, Dental Wax, Disinfectants, Preparations For Destroying Vermin, Fungicides, Herbicides
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 05.
[Class : 5] Pharmaceuticals, Medicinal Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] "Pharmaceutical, Veterinary And Sanitary
Preparations; Dietetic Substances Adapted For Medical Use, Food For Babies; Plasters, Materials For Dressings; Material For Stopping Teeth, Dental Wax; Disinfectants; Preparations For. Destroying Vermin; Fungicides, Herbicides".
Request for amendment is Pending for processing
[Class : 5] Pharmaceuticals, Medical Preparations And Fungicidal Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations; Sanitary Preparations For Medical Purposes; Pharmaceutical Creams.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations; Sanitary Preparations For Medical Purposes; Dietetic Food And Substances Adapted For Medical Or Veterinary Use, Food For Babies; Dietary Supplements For Human Beings And Animals; Plasters, Materials For Dressings; Material For Stopping Teeth, Dental Wax; Disinfectants; Preparations For Destroying Vermin; Fungi...
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations; Sanitary Preparations For Medical Purposes; Dietetic Food And Substances Adapted For Medical Or Veterinary Use, Food For Babies; Dietary Supplements For Human Beings And Animals; Plasters, Materials For Dressings; Material For Stopping Teeth, Dental Wax; Disinfectants; Preparations For Destroying Vermin; Fungi...