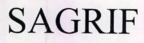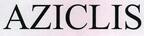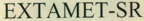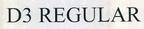Company Information
CIN
Status
Date of Incorporation
06 April 2005
State / ROC
Mumbai / ROC Mumbai
Industry
Sub Category
Non-govt company
Last Balance Sheet
Last Annual Meeting
Paid Up Capital
17,870,000
Authorised Capital
17,870,000
Past Directors
Patents
ABSTRACT
STABLE PHARMACEUTICAL COMPOSITION OF CARBETOCIN
The present invention provides a stable pharmaceutical compositions of Carbetocin. The present invention also provides a stable pharmaceutical composition of Carbetocin comprising, a therapeutically effective amount of Carbetocin, auxiliary agent, an iso...
ABSTRACT
“A room temperature stable aqueous solution for injection of Clevidipine”
The present invention provides a room temperature stable aqueous solution for injection comprising Clevidipine and solubilizer selected from Vitamin E polyethylene glycol succinate, propylene glycol, polyethylene glycol, or a mi...
A solid oral pharmaceutical composition comprising Rivaroxaban or a pharmaceutically acceptable salt or solvate thereof, one or more diluent, one or more binding agent, disintegrant and other pharmaceutically acceptable excipients wherein the composition is free of wetting agent. A process of preparing a solid oral ...
ABSTRACT
AN INTRAVENOUS INFUSION OF MAGNESIUM SULPHATE
The present invention is an intravenous infusion of magnesium sulphate. The present invention also an intravenous infusion of magnesium sulphate comprising magnesium sulphate, atleast two pH adjusting agents and a suitable vehicle. The present invention al...
“A room temperature stable solution for injection of Abaloparatide”
The present invention provides a room temperature stable solution for injection comprising Abaloparatide and stabilizer selected from L – methionine, disodium EDTA, D-?-tocopheryl polyethylene glycol succinate, hydroxypropyl betadex, mannitol, S-...
The present invention provides a stable topical composition comprising Tofacitinib or a pharmaceutically acceptable salt thereof, wherein the topical composition is lotion or gel, wherein the topical composition is stable for at least 6 months when stored at 30 °C temperature and 75% relative humidity. Further, th...
“A room temperature stable solution for injection of Abaloparatide”
The present invention provides a room temperature stable solution for injection comprising Abaloparatide and stabilizer selected from L – methionine, disodium EDTA, D-?-tocopheryl polyethylene glycol succinate, hydroxypropyl betadex, mannitol, S-...
Trademarks
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
[Class : 5] Medicinal &Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical And Trading In Medical
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal &Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticals Preparations Being Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Inlcuded In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Inlcuded In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Manufacturing And Trading In Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 3] Soaps; Essential Oils, Cosmetics, Hair Lotions, Dentifrices
[Class : 5] Medicinal And Pharmaceutical Preparation
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Clas 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 10] Feeding Bottle In Class 10
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medical And Pharmaceuticals Preparations Being Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations.
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations;
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Inlcuded In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparation Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Inlcuded In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Manufacturing And Trading In Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Application form not digitised
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 32] Manufacturing And Trading Mineral And Aerated Water, Other Non Alcoholic Drinks Energy Drink In Class 32
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Application form not digitised
[Class : 5] Medicinal And Pharmaceuticals Preparations Being Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceuticla Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations,
[Class : 5] Medicinal And Pharmaceutical Preparations,
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Manufacturing And Trading Of Meadicinal And Pharmaceitucal Preparations,
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparatios Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 05.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparation Included In Class 05.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
Application form not digitised
[Class : 5] Medicinal And Pharmaceutical Preparations Included In Class 5.
[Class : 5] Medicinal And Pharmaceutical Preparations Inlcuded In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceuticla Preparations Included In Class 5.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Sterile Hydrogel Surgical Dressing Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Madicinal And Pharmaceutcal Prepaeation.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 10] Condom
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparation
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Manufacturing And Trading In Medicinal And Pharmaceutical Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Medicinal And Pharmaceutical Preparations In Class 05
[Class : 1] Excipient Agent And Artificial Sweeteners Which Are Included In Class 01
[Class : 1] Excipient Agent And Artificial Sweeteners Which Are Included In Class 01
[Class : 1] Excipient Agent And Artificial Sweeteners Which Are Included In Class 01
[Class : 5] Pharmaceutical, Veterinary And Sanitary Preparations; Dietetic Substances Adapted For Medical Use, Food For Babies; Plasters, Materials For Dressings; Materials For Stopping Teeth, Dental Wax; Disinfectants; Preparation For Destroying Vermin; Fungicides, Herbicides
[Class : 5] Pharmaceutical, Veterinary And Sanitary Preparations; Dietetic Substances Adapted For Medical Use, Food For Babies; Plasters, Materials For Dressings; Materials For Stopping Teeth, Dental Wax; Disinfectants; Preparation For Destroying Vermin; Fungicides, Herbicides