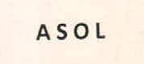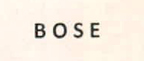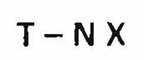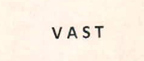Company Information
CIN
Status
Date of Incorporation
20 January 1995
State / ROC
Ahmedabad / ROC Ahmedabad
Industry
Sub Category
Non-govt company
Last Balance Sheet
Last Annual Meeting
Paid Up Capital
200,297,280
Authorised Capital
320,000,000
Directors
Past Directors
Patents
Discloses a novel liquid rectal spray dosage form comprising Diclofenac or its pharmaceutically active salts, in unique blend of solvents and co-solvents, for the treatment of pre- and post-operative pain, gynecological surgery and musculoskeletal disorders such as muscular pain, pain associated with arthritis, acut...
Disclosed herein is a liquid oral spray composition comprising combination of antiallergic, cough suppressant and nasal decongestant useful for management of dry or non-productive cough, cough associated with viral or bacterial inflammation of upper respiratory tract, sinusitis, laryngitis, tracheitis and pharyngiti...
Disclosed herein is liquid oral spray composition comprising Thiocolchicoside in combination with at least one anti-inflammatory agent in unique blend of solvents alongwith pharmaceutically acceptable excipients useful for pain management in arthritis, rheumatoid arthritis, acute nonspecific low back pain, osteoporo...
Disclosed herein is an oral spray composition comprising donepezil hydrochloride intended to increase the oral bioavailability and to bypass hepatic metabolism.
Disclosed herein is stable pharmaceutical composition comprising an active ingredient diclofenac sodium with other pharmaceutical excipients. It relates to a quick and efficient intravenous injectable formulation which is useful for the treatment of pain.
The present invention relates to a novel pharmaceutical compostion for the treatment of allergic rhinitis. More particularly, the present invention relates to a novel pharmaceutical composition containing anti-inflammatory, anti-histaminic and decongestant ingredients for sustained release in the treatment of allerg...
A Novel Nasal Drug Delivery System Of On Dansetron Hydrochloride And Process For Preparation Thereof
The present invention relates to novel nasal drug delivery system of Ondansetron Hydrochloride in form of nasal spray and process for preparation thereof. The nasal mucosa is more sensitive to external influences, large and easily accessible area of highly permeable, vascularized tissue through which drugs absorbed ...
The present invention relates to a novel mouth melting pharmaceutical dosage film comprising medicaments and process for preparation thereof. The dosage is Compact, Convenient, Discrete, Effective, Fast, Metered, With Great taste, Advanced technology and accurate than the existing / conventional formulation.
The ob...
The present invention relates to a buffered novel antiseptic combination of Povidone Iodine and formulation thereof. The present a buffered novel antiseptic combination of Povidone Iodine and Triclosan is synergistic to provide long duration of activity by long cumulative residual microbial activities. The formulati...
The oromucosal solution formulation comprises of a local anaesthetic and a debriding agent used for the treatment of ulcerating oral mucosal lesions known as Aphthous stomatitis or Canker Sores. The formulation contains non-saccharide and zero calorie sweeteners, thus having an appeal to diabetics and prevents tooth...
Malaria is a vector borne infectious disease caused by protozoan parasites.
Oil based arteether injections are used as anti-malarial drugs, specifically for the
treatment of chloroquine resistant Plasmodium falciparum malaria and cerebral
malaria cases. Arteether is a fast acting blood schizontocide. The injec...
Disclosed herein a liquid vaginal spray composition comprising natural/ synthetic progesterone along with unique blend of solvent/co-solvent and anti-oxidants useful for treating secondary amenorrhea, threatened or habitual abortions and infertility.
The present invention relates to a novel oral liquid dosage form composition of paracetamol alone or paracetamol in combination with nasal decongestant, and anti-allergic agent and process of preparation thereof. More particularly present invention includes a novel oral liquid dosage form composition containing para...
The present invention relates to formulation containing aceclofenac and methionine in which methionine (its isomers and/or racemic mixture) prevents the hepatototic effects of aceclofenac. Methionine also revitalizes the liver in case of hepatotoxicity of aceclofenac. The oral dosage forms containing Methionine and ...
The present invention relates to formulation containing Diclofenac and methionine in which methionine (its isomers and/or racemic mixture) prevents the hapatototic effects of Diclofenac. Methionine also revitalizes the liver in case of hepatotoxicity of Diclofenac. The oral dosage forms containing Methionine and Dic...
The present invention discloses a pharmaceutical topical drug applicator which comprises cylindrical plastic housing of container on top of it socket is located in to which a roll on ball is fitted. Plastic housing of container (3) is having concavity on the one side. This applicator can be uses for managing the are...
This invention relates to pharmaceutical composition of Misoprostol for suppository dosage form and method of preparation thereof. It gives synergistic effect in the gynecological problems, including cervical ripening, labor induction, mid-trimester terminations and postpartum hemorrhage. Oily based suppository form...
The present invention relates to a pharmaceutical formulation containing high concentration of Diclofenac (or its salts) injection that provides ease of administration to the patient. Diclofenac and its pharmaceutically acceptable salts in higher concentration (75 mg/ml) injectable dosage form results in unique pain...
The present invention refers to a pharmaceutical formulation for paracetamol injection which comprises paracetamol, glycofurol, co-solvent, water and preservative. This formulation provides ease of administration to the patient, less pain to the patient and high effectiveness. The said formulation is ready to use fo...
The present invention relates to a pharmaceutical composition for the treatment of allergic rhinitis comprising non-steroidal anti-inflammatory drug, antihistamine, decongestant, methionine, one or more conventional additives and pharmaceutical acceptable excipients. The invention is to provide pharmaceutical formul...
Disclosed herein is liquid vaginal spray dosage form to provide local as well as systemic effect, comprising therapeutically effective amount of at least one antifungal agent along with lactic acid, useful for the treatment of vaginal fungal infections like vulvovaginal Candidiasis or "yeast" infection bacterial vag...
Disclosed herein is a high concentrated liquid rectal spray composition comprising Bisacodyl and its pharmaceutically acceptable salts in unique blend of solvent, mucoadhesive polymers and preservatives; along with pharmaceutically acceptable excipients, useful for the treatment of constipation and bowel evacuation ...
The present invention provides a novel pharmaceutical composition for the treatment of pain and inflammation containing nimesulide, a muscle relaxant methionine, one or more conventional additives and pharmaceutical acceptable excipients. The invention is to provide pharmaceutical formulation for treatment of pain a...
This invention is based on an injectable formulation which comprises Ofloxacin along with two or more active ingredients along with some pharmaceutically acceptable excipients for the treatment of infections caused due to both gram positive and gram negative bacteria.
Invention provides the formulation containing Methionine and Nimesulide in which Methionine (its isomers and/or racemic mixture) prevents the hepatotoxic effects of Nimesulide. Methionine also revitalizes the liver in case of hepatotoxicity of Nimesulide. The oral dosage forms containing Methionine and Nimesulide to...
Trademarks
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical & Medicinal Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 05.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Application form not digitised
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations
Request for amendment is Pending for processing
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceuticals, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceutical And Mecdicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Medicinal Preparations Containing Paracetamol.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 05.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Mecdicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Division, Medicinal And Pharmaceutical Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparationas.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 05.
[Class : 5] Pharmaceuticals And Medicinal Preparations Included In Class 05.
Application form not digitised
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparation
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Request for amendment is Pending for processing
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinals Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations Included In Class 05.
Application form not digitised
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceutical Preparations Included In Class 05.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Harmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharamceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
Request for amendment is Pending for processing
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medical Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations Included In Class 05.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations Included In Class 5.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical, Veterinary, Sanitary, And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparation
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical, Veterinary, Sanitary And Medicinal Preparations.
Request for amendment is Pending for processing
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations Included In Class 05.
[Class : 5] Pharmaceutical And Mecdicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparation, Sanitary Preparation For Medical Purpose, Dietetic Food And Substances Adapted For Medical Or Veterinary Use, Food For Babies, Dietary Supplements For Humans And Animals, Plasters, Materials For
Dressings, Material For Stopping Teeth, Dental Wax Disinfectants, Preparation For Destroying Vermin, Fungicides, Hed...
[Class : 5] It.S Product Contain Vitamin C & Zinc
[Class : 5] Pharmaceuticals And Medical Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical Preparations And Medicinal
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
Request for amendment is Pending for processing
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 3] Pharmaceutical Medical Cosmetic Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Medical And Pharmaceutical Preparations In Class 5
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical & Medical Preparations In Class 5
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 05.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical Preparations.
[Class : 5] Pharmaceutical Preparations Included In Class 05.
[Class : 5] Pharmaceutical Preparations Included In Class 05.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medical Preparations In Class 5
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical & Medical Preparations In Class 5
[Class : 5] Pharmaceutical & Medicinal Preparations & Substances
Awaiting Reply to Examination Report
[Class : 5] Medical And Pharmaceutical Preparations In Class 5
[Class : 5] Medical And Pharmaceutical Preprations In Class 5
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical & Medical Preparations In Class 5
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutial Preparations
[Class : 5] Pharmaceutical And Medical Preparations In Class 5
[Class : 5] Pharmaceutical And Medical Preparation In Class 5
[Class : 5] Pharmaceutical And Medical Preparations In Class 5
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Pharmaceutical And Medical Preparation Diet Supplimentary
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical And Medical Preparations
[Class : 5] Medical & Pharmaceutical Preparations
[Class : 5] Medical And Pharmaceutical Preparations
[Class : 5] Pharmaceutical & Medical Preparations In Management Of Mixed,Progressive & Conductive Hearing Loss,Otosclerosis
[Class : 5] Preparation Of Medical And Pharmaceutical In The Management Of Mixed,Progressive & Conductive Hearing Loss,Otosclerosis
[Class : 5] Medical & Pharmaceutical Preparation Clinically Proven For Effective Care
[Class : 5] Medical & Pharmaceutical Preparations For Cognitive Function Ent Product
[Class : 5] Pharmaceutical Formulations For Medical Preparations
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”
[Class : 5] Medications Formulated To Alleviate, Manage, Or Reduce The Perception And Severity Of Tinnitus Symptoms”