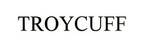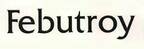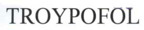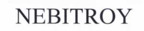Company Information
CIN
Status
Date of Incorporation
17 February 1999
State / ROC
Ahmedabad / ROC Ahmedabad
Industry
pharmaceuticals: antibiotics, endocrine products
Sub Category
Non-govt company
Last Balance Sheet
Last Annual Meeting
Paid Up Capital
86,872,480
Authorised Capital
100,000,000
Directors
Past Directors
Patents
The present invention relates to advanced topical formulations of pharmaceutically acceptable salts of Heparin providing enhanced transdermal penetration. The present invention provides clear, non-sticky liquid formulations in which the drug is ready-for-absorption and which are suitable for administration in the fo...
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150mg/ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent ...
Vitamin B-12 deficiency is very common among population. The main causes of B-12 deficiency include lack of intrinsic factors and other intestinal factors (e.g. malabsorption), rare genetic disorders, and inadequate intake. The present invention relates to intranasal formulations of vitamin B derivatives such as met...
The condition wherein the stomach contains an excessive amount of hydrochloric acid is called as hyperacidity and is often associated with other conditions such as flatulence. The present invention provides formulations of antacids in combination with anti-flatulents suitable for the treatment of such conditions of ...
The present invention provides a pharmaceutical composition comprising angiotensin II receptor antagonist telmisartan and process for preparing the same, wherein said composition devoid of use of any surfactants.
Disclosed herein a fluid dispensing massage device capable of being dispense desired fluid and massaging the concerned area using said device without dispensing additional fluid. The device comprising an upstanding body with hollow interior defining a central vertical axis, a neck portion having an open mouth, a hol...
Disclosed herein is a non-gastro retentive composition comprising a therapeutically effective amount of methylcobalamin or its pharmaceutically acceptable forms and process for preparing the same, wherein said composition provide sustained blood levels of methylcobalamin for several hours i.e. for 24 hours without h...
Disclosed herein are injectable compositions containing high concentration of paracetamol or its pharmaceutically acceptable salts wherein the concentration of paracetamol or its pharmaceutically acceptable salt is >150mg/ml in a judiciously tailored solvent system comprising glycofurol, ethanol, water or a solvent ...
Oromucosal Solutions of Zolpidem or Pharmaceutically acceptable Salts thereof
The present invention relates to buccal or sublingual formulations of Zolpidem or pharmaceutically acceptable salt thereof. The formulations minimize the amount of penetration enhancers and yet provide rapid transmucosal penetration of th...
Oromucosal Solutions of Zolpidem or Pharmaceutically acceptable Salts thereof
The present invention relates to buccal or sublingual formulations of Zolpidem or pharmaceutically acceptable salt thereof. The formulations minimize the amount of penetration enhancers and yet provide rapid transmucosal penetration of th...
Trademarks
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements,
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietry Supplements
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes; Printed Matier; Bookbinding Material; Photographs; Stationery; Adhesives For Stationery Or Household Purposes; Artists' Materials; Paint Brushes; Typewriters And Office Requisites (Except Furniture); Instructional And Teaching Material (Except Apparatus); Plastic Materials For P...
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 35] Advertising, Business Management, Business Administration, Office Functions
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietry Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 35] Advertising, Business Managenient, Business Administration, Office Functions
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparation.
[Class : 5] Medicinal, Pharmaceutical And Veterinary Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes, Printed Matier, Bookbinding Material, Photographs, Stationery, Adhesives For Stationery Or Household Purposes, Artists Materials, Paint Brushes, Typewriters And Office Requisites (Except Furniture), Instructional And Teac Hl Ng Material (Except Apparatus), Plastic Materials For ...
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Renewal of trademark is overdue. Renewal with Restoration request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pahrmaceuticals Medicinal Preparations And Dietary Supplemetns
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparation.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Medicinal And Pharmaceutical Preparations
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes; Printed Matter; Bookbinding Material; Photographs; Stationery; Adhesives For Stationery Or Household Purposes; Artists' Materials; Paint Brushes; Typewriters And Office Requisites (Except Furniture); Instructional And Teaching Material (Except Apparatus); Plastic Materials For P...
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Prepartions.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticlas Medicinal Preparations And Dietary Supplements
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical; Veterinary And Sanitary Substances; Infants And Invalids Foods, Plasters, Materials For Bandaging; Materials For Stopping Teeth, Dental Wax; Disinfectants; Preparations For Killing Weeds And Destroying Vermin.
Application form not digitised
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations, Included In Class 5.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Phramceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparation.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparation.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes; Printed Matier; Bookbinding Material; Photographs; Stationery; Adhesives For Stationery Or Household Purposes; Artists' Materials; Paint Brushes; Typewriters And Office Requisites (Except Furniture); Instructional And Teaching Material (Except Apparatus); Plastic Materials For P...
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Renewal of trademark is overdue. Renewal with Restoration request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparatins
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceuticals And Medicinal Preparations
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Renewal of trademark is overdue. Renewal with Restoration request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 35] Advertising, Business Managenient, Business Administration, Office Functions
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Midicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Phramceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations Included In Class 5.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceutical And Medicinal Preparations.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Phramceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preparations For Use As Anti Spasmodic Medicine.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 24] Textiles And Textile Goods, Not Included In Other Classes, Bed And Table Covers.
[Class : 25] Clothing, Footwear, Headgear.
[Class : 26] Lace And Embroidery, Ribbons And Braid, Buttons, Hooks And Eyes, Pins And Needles, Artificial Flowers.
[Class : 27] Carpets, Rugs, Mats And Matting, Linoleum And Other Materials For Covering Existing Floors, Wall Hangings (Non Textile).
[Class : 28] Games And Playthings, Gymnastic And Sporting Articles Not Included In Other Classes, Decorations For Christmas Trees.
[Class : 29] Meat, Fish, Poultry And Game, Meat Extracts, Preserved, Dried And Cooked Fruits And Vegetables, Jellies, Jams, Fruit Sauces, Eggs, Milk And Milk Products, Edible Oils And Fats.
[Class : 30] Coffee, Tea, Cocoa, Sugar, Rice, Tapioca, Sago, Artificial Coffee, Flour And Preparations Made From Cereals, Bread, Pastry And Confectionery, Ices, Honey, Treacle, Yeast, Baking Powder, Salt, Mustard, Vinegar, Sauces, (Condiments), Spices, Ice.
[Class : 31] Agricultural, Horticultural And Forestry Products And Grains Not Included In Other Classes, Live Animals, Fresh Fruits And Vegetables, Seeds, Natural Plants And Flowers, Foodstuffs For Animals, Malt.
[Class : 32] Beers, Mineral And Aerated Waters, And Other Non Alcoholic Drinks, Fruit Drinks And Fruit Juices, Syrups And Other Preparations For Making Beverages.
[Class : 33] Alcoholic Beverages (Except Beers).
[Class : 34] Tobacco, Smokers Articles, Matches.
[Class : 35] Advertising, Business Management, Business Administration, Oflice Functions.
[Class : 36] Insurance, Financial Affairs, Monetary Affairs, Real Estate Affairs.
[Class : 37] Building Construction, Repair, Installation Services.
[Class : 38] Providing Services Of Telecommunication Channels For Teleshopping, Connection & User
Access To Computer Network, Computer Terminals, Video Conferencing, Internet Chatrooms,
Online Forums & Rental Of Modems, Computer Aided Transmission Of Messages, Images, Paging & Database To Bring Together And Enabling The Customers To View, ‘Talk And Visual
Communication Wi...
[Class : 39] Transport, Packaging And Storage Of Goods, Travel Arrangement.
[Class : 40] Treatment Of Materials.
[Class : 41] Education, Providing Of Training, Entertainment, Sporting And Cultural Activities.
[Class : 42] Scientific And Technological Services And Research And Design Relating Thereto, Industrial Analysis And Research Services, Design And Development Of Computer Hardware And Software.
[Class : 43] Services For Providing Food And Drink, Temporary Accommodation.
[Class : 44] Medical Services, Veterinary Services, Hygienic And Beauty Care For Human Beings Or Animals, Agriculture, Horticulture And Forestry Services.
[Class : 45] Legal Services, Security Services For The Protection Of Property And Individuals, Personal And Social Services Rendered By Others To Meet The Needs Of Individuals.
[Class : 1] Chemicals Used In Industry, Science And Photography, As Well As In Agriculture, Horticulture And Forestry, Unprocessed Artificial Resins, Unprocessed Plastics, Manures, Fire Extinguishing Compositions, Tempering And Soldering Preparations, Chemical Substances For Preserving Foodstuffs, Tanning Substances, Adhesives Used In Industry.
[Class : 2] Paints, Varnishes, Lacquers, Preservatives Against Rust And Against Deterioration Of Wood, Colorants, Mordents, Raw Natural Resins, Metals In Foil And Powder Form For Painters, Decorators, Printers And Artists.
[Class : 3] Bleaching Preparations And Other Substances For Laundry Use, Cleaning, Polishing, Scouring And Abrasive Preparations, Soaps, Perfumery, Essential Oils, Cosmetics, Hair Lotions, Dentifrices.
[Class : 4] Industrial Oils And Greases, Lubricants, Dust Absorbing, Wetting And Binding Compositions, Fuels (Including Motor Spirit) And Illuminants, Candles, Wicks.
[Class : 6] Common Metals And Their Alloys, Metal Building Materials, Transportable Buildings Of Metal, Materials Of Metal For Railway Tracks, Non Electric Cables And Wires Of Common Metal, Ironmongery, Small Items Of Metal Hardware, Pipes And Tubes Of Metal, Safes, Goods Of Common Metal Not Included In Other Classes, Ores.
[Class : 7] Machines And Machine Tools, Motors And Engines (Except For Land Vehicles), Machine Coupling And Transmission Components (Except For Land Vehicles), Agricultural Implements Other Than Hand Operated, Incubators For Eggs.
[Class : 8] Hand Tools And Implements (Hand Operated), Cutlery, Side Arms, Razors.
[Class : 9] Scientific, Nautical, Surveying, Electric, Photographic, Cinematographic, Optical, Weighing, Measuring, Signalling, Checking (Supervision), Life Saving And Teaching Apparatus And Instruments, Apparatus For Recording, Transmission Or Reproduction Of Sound Or Images, Magnetic Data Carriers, Recording Discs, And Mechanisms For Coin Operated Apparatus, Cash Registers...
[Class : 10] Surgical, Medical, Dental And Veterinary Apparatus And Instruments, Artificial Limbs, Eyes And Teeth, Orthopaedic Articles, Suture Materials.
[Class : 11] Apparatus For Lighting, Heating, Steam Generating, Cooking, Refrigerating, Drying Ventilating, Water Supply And Sanitary Purposes.
[Class : 12] Vehicles, Apparatus For Locomotion By Land, Air Or Water.
[Class : 13] Firearms, Ammunition And Projectiles, Explosives, Fireworks.
[Class : 14] Precious Metals And Their Alloys And Goods In Precious Metals Or Coated Therewith, Not Included In Other Classes, Jewellery, Precious Stones, Horological And Other Chronometric Instruments.
[Class : 15] Musical Instruments.
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes, Printed Matter, Bookbinding Material, Photographs, Stationery, Adhesives For Stationery Or Household Purposes, Artists Materials, Paint Brushes, Typewriters And Office Requisites (Except Furniture), Instructional And Teaching Material (Except Apparatus), Plastic Materials For Pa...
[Class : 17] Rubber, Gutta Percha, Gum, Asbestos, Mica And Goods Made From These Materials And Not Included In Other Classes, Plastics In Extruded Form For Use In Manufacture, Packing, Stopping And Insulating Materials, Flexible Pipes, Not Of Metal.
[Class : 18] Leather And Imitations Of Leather, And Goods Made Of These Materials And Not Included In Other Classes, Animal Skins, Hides, Trunks And Travelling Bags, Umbrellas, Parasols And Walking Sticks, Whips, Harness And Saddlery.
[Class : 19] Building Materials, (Non Metallic), Non Metallic Rigid Pipes For Building, Asphalt, Pitch And Bitumen, Nonmetallic Transportable Buildings, Monuments, Not Of Metal.
[Class : 20] Furniture, Mirrors, Picture Frames, Goods (Not Included In Other Classes) Of Wood, Cork, Reed, Cane, Wicker, Horn, Bone, Ivory, Whalebone, Shell, Amber, Mother Of Pearl, Meerschaum And Substitutes For All These Materials, Or Of Plastics.
[Class : 21] Household Or Kitchen Utensils And Containers (Not Of Precious Metal Or Coated Therewith), Combs And Sponges, Brushes(except Paints Brushes), Brush Making Materials, Articles For Cleaning Purposes, Steelwool, Unworked Or Semi Worked Glass (Except Glass Used In Building), Glassware, Porcelain And Earthenware Not Included In Other Classes.
[Class : 22] Ropes, String, Nets, Tents, Awnings, Tarpaulins, Sails, Sacks And Bags (Not Included In Other Classes) Padding And Stuffing Materials (Except Of Rubber Or Plastics), Raw Fibrous Textile Materials.
[Class : 23] Yarns And Threads, For Textile Use.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals And Medicinal Preparations For The Treatment Of High Triglyceride Levels.
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals And Medicinal Preparations
[Class : 5] In Respect Of Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 35] Advertising, Business Management, Business Administration, Office Functions Related To Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements In Class 5
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is overdue. Renewal request with surcharge in prescribed form is to be filed by the applicant.
[Class : 35] Advertising, Business Management, Business Administration, Office Functions.
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes, Printed Matter, Bookbinding Material, Photographs, Stationery, Adhesives For Stationery Or Household Purposes, Artists Materials, Paint Brushes, Typewriters And Office Requisites (Except Furniture), Instructional And Teaching Material (Except Apparatus), Plastic Materials For Pa...
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Renewal of trademark is overdue. Renewal with Restoration request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 16] Paper, Cardboard And Goods Made From These Materials, Not Included In Other Classes; Printed Matter; Bookbinding Material; Photographs; Stationery; Adhesives For Stationery Or Household Purposes; Artists' Materials; Paint Brushes; Typewriters And Office Requisites (Except Furniture); Instructional And Teaching Material (Except Apparatus); Plastic Materials For P...
[Class : 35] Advertising, Business Managenient, Business Administration, Office Functions
[Class : 35] Advertising, Business Management, Business Administration, Office Functions
[Class : 5] Pharmaceutical And Medicinal Preparations.
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary
Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements In Class 5
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparation
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] “Oromucosal Mouth Sprays Containing
Zolpidem
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
Awaiting Reply to Examination Report
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations.
[Class : 5] Pharmaceuticals, Medicinal And Veterinary Preparations.
[Class : 5] Pharmaceuticals, Medicinal And Veterinary Preparations.
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Medicinal And Pharmaceutical Products; Veterinary Products; Disinfectants And Antiseptics; Sanitizers;
[Class : 5] Pharmaceuticals, Medicinal And Veterinary Preparations
[Class : 5] Medicinal And Pharmaceutical Preparations; Nutritional Preparations; Nutraceutical Preparations; Dietary Preparations;
[Class : 5] Medicinal And Pharmaceutical Preparations; Sanitary Preparation; Disinfectants; Antiseptics; Sanitizers; Veterinary Preparations;
[Class : 5] Pharmaceuticals, Medicinal And Veterinary Preparations
[Class : 42] Design And Development Of Computer Software;
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medicinal & Veterinary Preparations.
[Class : 5] Pharmaceuticals, Medicinal & Veterinary Preparations.
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceuticals And Medicinal Preparations.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
Trade mark is likely to be removed due to non filing of Renewal request within prescribed time limit In case of any discripancy contact respective TM Registry.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is overdue. Renewal with Restoration request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
Renewal of trademark is due. Renewal request in prescribed form is to be filed by the applicant.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals Medicinal Preparations And Dietary Supplements.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Medicinal & Pharmaceutical Preparations Included In Class 5.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations.
[Class : 5] Pharmaceutical And Medicinal Preparations
[Class : 5] Pharmaceutical And Medicinal Preprations, Included In Class 5.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal & Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary
Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations.
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
Ready for Show cause Hearing
[Class : 5] Pharmaceuticals, Medical And Veterinary, Preparations.
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
Request for amendment is Pending for processing
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceuticals, Medical And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary
Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary
Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary
Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And
Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations
[Class : 5] Pharmaceutical, Medicinal And Veterinary Preparations